Online TestScience
Carbon and its Compounds
Carbon and its Compounds
Congratulations - you have completed Carbon and its Compounds.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
The force of attraction that exists between the carbon atoms in graphite is ................
strong electrostatic forces of attraction | |
strong vander Waals force of attraction | |
weak vander Waals force of repulsion | |
weak vander Waals force of attraction |
Question 2 |
Carbon compounds are stable due to ......................
its electronic configuration | |
its tendency to show isomerism | |
its small size | |
its ability to combine with non metals |
Question 3 |
Choose the correct statement ................
The tendency of carbon atom to form covalent bonds with other carbon atoms is known as catenation | |
Carbon can form C+4 and C-4 ions | |
The valency of carbon is always four | |
The hardness of diamond is due to the weak vander waals force that exist between carbon atoms |
Question 4 |
Which of the following form a set of homologous series?
ethane, methane, propene | |
ethane, methane, ethane | |
ethyne, propyne, but-l-yne | |
ethyne, propyne, but-l-ene |
Question 5 |
Which of the following represent the functional group ‘alcohols’ ...................
–CHO | |
CO | |
CHO | |
COOH |
Question 6 |
The IUPAC name for CH3COCH3 is ....................
acetone | |
dimethyl ketone | |
propanone | |
propane |
Question 7 |
A compound has the molecular formula C2H6O. The functional group present in the compound is ....................
–OH | |
CO | |
Cho | |
COOH |
Question 8 |
Which of the following pairs of compounds are isomers?
ethane and methane | |
ethyne and propyne | |
but-l-ene and but-2-ene | |
acetylene and ethyne |
Question 9 |
Four pairs of compounds are given below. Choose the pairs of compounds containing the same functional group ...................
Ethanol and ethanol | |
propane and propyne | |
propanol and propanoic acid | |
Methanol and butanol |
Question 10 |
The IUPAC name for acetic acid is ......................
methanoic acid | |
ethanoic acid | |
propanoic acid | |
butanoic acid |
Question 11 |
The conversion of ethanol to ethane is ..................
inter molecular dehydration | |
intra molecular dehydration | |
inter molecular hydration | |
oxidation |
Question 12 |
CH3CH2OH CH3CHO + H2. This reaction is known as ...................
esterification | |
dehydration | |
dehydrogenation | |
oxidation |
Question 13 |
An example for decarboxylation reaction is ...............
heating ethanoic acid with sodium hydroxide | |
heating sodium ethanoate with soda lime | |
treating glucose with zymase | |
heating ethanol with sodium |
Question 14 |
Which of the following on heating liberate hydrogen gas?
- ethanol, 2. Acetic acid, 3. Methane, 4. Acetone
1 only | |
2 only | |
1 and 2 only | |
3 and 4 |
Question 15 |
Choose the alkyne from the following ..............
methane | |
ethane | |
ethane | |
acetylene |
Question 16 |
Which of the following represent a pair of unsaturated hydrocarbon?
ethane and ethyne | |
ethene and ethyne | |
methane and ethyne | |
acetic acid and formic acid |
Question 17 |
An organic compound decolourises the bromine water. It may be .................
ethane | |
ethane | |
propane | |
butane |
Question 18 |
Rectified spirit’ contains ....................
95% methanol and 5% water | |
95% ethanol and 5% water | |
15% ethanol and 95% water | |
50% ethanol and 50% water |
Question 19 |
On heating ethanol with conc. H2SO4 at 443 K gives ............
ethane | |
ethane | |
ethyne | |
methane |
Question 20 |
Molasses contain nitrogenous matter. If the nitrogen content of the molasses is poor during fermentation ....................... is added to fortify.
ammonium chloride | |
ammonium sulphate | |
yeast | |
sulphyric acid |
Question 21 |
Ethanol on oxidation in the presence of alkaline potassium permanganate or acidified potassium dichromate gives the following acid ....................
propanoic acid | |
butanoic acid | |
methanoic acid | |
ethanoic acid |
Question 22 |
The functional group of carboxylic acid is .....................
–OH | |
–CHO | |
>C = O | |
–COOH |
Question 23 |
The term ‘organic chemistry’ was first used by the Swedish chemist ...................
Priestly | |
Berzelius | |
Hall | |
Dewer |
Question 24 |
The scientist who first prepared urea from an inorganic compound ammonium cyanate is ..................
Wohler | |
Brovon | |
Berzelius | |
Mendeleev |
Question 25 |
Kohinor diamond is a .................... carat diamond.
24 | |
105 | |
128 | |
22 |
Question 26 |
Fullerene consists of ....................... carbon atoms.
4 | |
32 | |
48 | |
60 |
Question 27 |
Ethanol when reacts with sodium gives .......................... gas.
hydrogen | |
methane | |
oxygen | |
stearm |
Question 28 |
Percentage of sucrose in molasses is ......................
40% | |
60% | |
25% | |
30% |
Question 29 |
The method of preparation of alcohol from molasses is .......................
esterification | |
fermentation | |
polymerisation | |
reduction |
Question 30 |
The enzyme which helps in the conversion of glucose to ethanol is ................
sucrose | |
zymase | |
invertase | |
kirase |
Question 31 |
.................... is a good conductor of electricity unlike other non-metals.
Diamond | |
Graphite | |
Nitrogen | |
Carbon |
Question 32 |
Carbon compounds have low melting and boiling points because of the ........................ nature
covalent | |
ionic | |
coordinate | |
metallic |
Question 33 |
The chemical properties of the members of the homologous series are ...................
different | |
similar | |
various | |
not similar |
Question 34 |
The IUPAC name of the compound CH3 CH2 CH3 is ...................
methane | |
propane | |
n-butane | |
butane |
Question 35 |
ROH is alkanol, therefore, CH3 CH2 OH is ........................
methanol | |
propanol | |
ethanol | |
ethanol |
Question 36 |
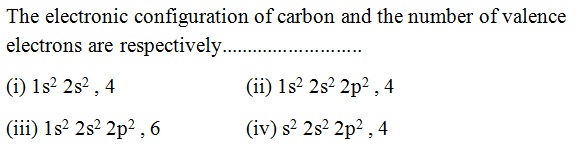
A | |
B | |
C | |
D |
Question 37 |
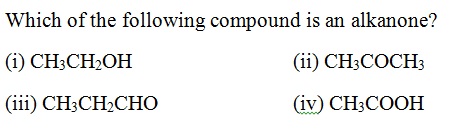
A | |
B | |
C | |
D |
Question 38 |

A | |
B | |
C | |
D |
Question 39 |
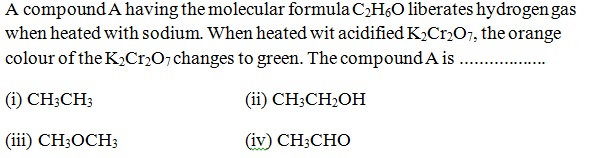
A | |
B | |
C | |
D |
Question 40 |
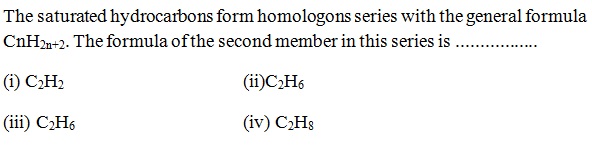
A | |
B | |
C | |
D |
Question 41 |

A | |
B | |
C | |
D |
Question 42 |

A | |
B | |
C | |
D |
Question 43 |
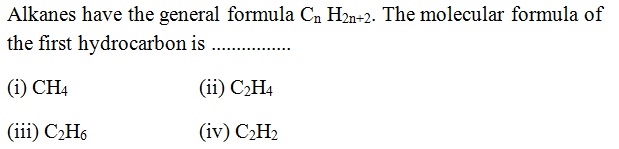
A | |
B | |
C | |
D |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
There are 43 questions to complete.