Online Test
Periodic Classification of Elements
Periodic Classification of Elements
Congratulations - you have completed Periodic Classification of Elements.
You scored %%SCORE%% out of %%TOTAL%%.
Your performance has been rated as %%RATING%%
Your answers are highlighted below.
Question 1 |
A plot of square rod of frequency of X-rays emitted by a metal against atomic number gives .................
a straight line | |
a curve | |
a straight line parallel to X axis | |
a straight line parallel to Y axis |
Question 2 |
The basis for periodic classification of the elements as suggested by Mosley is ........
atomic weight | |
valency | |
atomic number | |
physical and chemical properties |
Question 3 |
In the long form of periodic table.
- Elements have been arranged in increasing order of atomic weight.
- Elements have been arranged in increasing order of atomic number.
- There are seven periods and 18 groups
- There are eight periods and 19 groups
1,2,3 | |
2 and 3 | |
1, 3 | |
3 and 4 |
Question 4 |
The third period consists of elements presents having atomic number.
1,2 | |
8 | |
11 to 18 | |
19 to 36 |
Question 5 |
The maximum number of elements present in third period is ...............
2 | |
8 | |
18 | |
36 |
Question 6 |
The fourth period of the long form of periodic table.
contain eight elements | |
contains 8 normal elements, 10 transition elements and 14 inner transition elements | |
contains elements from rubidium to xenon | |
contains elements from potassium to krypton |
Question 7 |
Elements having atomic numbers 55 to 86 are present ..................
fourth period | |
fifth period | |
sixth period | |
seventh period |
Question 8 |
In the long form of periodic table alkali and alkaline earth metals are present in respectively .....................
first group and second group | |
second group and first group | |
in first group only | |
in second group only |
Question 9 |
Group 3 to 12 in the long form of periodic table are called .................
respective elements | |
transition elements | |
inner transition elements | |
inert gases |
Question 10 |
Choose the incorrect statement.
The chemical properties of element change along a period | |
The elements present in a group have identical chemical properties | |
The size of the atom increases along a period | |
The size of the atom increases along a group |
Question 11 |
...................... are called a coinage metals
Copper, Silver and Gold | |
Copper, Brass and Gold | |
Copper, Brass and Silver | |
Copper Silver and Aluminium |
Question 12 |
Which metal is a constitute of haemoglobin?
zn | |
Fe | |
Ca | |
Co |
Question 13 |
Given from pairs, identify the one which is an ore and the other a mineral.
Bauxite, Cryolite | |
Galena, Zinc blend | |
Haematic, Clay | |
Bauxite and Zinc blende |
Question 14 |
A metal A is not affected by dry air on heating to 800o it burns brightly. It is a powerful reducing agent. It is used in alumina thermic process. The metal A is ..............
Fe | |
Zn | |
Al | |
Cu |
Question 15 |
Matte is ................
a mixture of cuprous sulphide and ferrous sulphide | |
a mixture of cupric sulphide and ferrous sulphide | |
a mixture of cuprous sulphide and ferrous sulphate | |
a mixture of cupric sulphide and ferrous sulphate |
Question 16 |
Copper metal is heated with concentrated nitric acid. The gas evolved is .............
nitrous oxide | |
nitric oxide | |
nitrogen dioxide | |
sulphurdioxide |
Question 17 |
Choose the correct statement from the following:
Haematite ore is concentrated by froth flotation process | |
Haematite ore is concentrated by gravity separation | |
Copper pyrites is concentrated by gravity separation | |
Haematite ore is concentrated by leaching |
Question 18 |
Which of the following metals is purified by electrolytic refining?
Fe | |
Cu | |
Zn | |
Hg |
Question 19 |
Number of periods in modern periodic table is ........................
7 | |
17 | |
18 | |
8 |
Question 20 |
An amalgam is an alloy of metal with .........................
carbon | |
hydrogen | |
mercury | |
gold |
Question 21 |
Atomic number of iron is 26. Its electronic configuration is ..................
2, 8, 8, 2 | |
2, 8, 8, 4 | |
2, 8, 14, 2 | |
2, 8, 14, 4 |
Question 22 |
Bauxite is used to extract aluminium. It can be turned as .................
ore | |
mineral | |
flux | |
slag |
Question 23 |
To design the body of the aircraft, ................... alloys are used.
iron | |
gold | |
silver | |
aluminium |
Question 24 |
A process employed for the concentration of sulphide ore is ....................
gravity separation | |
forth floatation | |
magnetic separation | |
chemical method |
Question 25 |
modern periodic law states that the physical and chemical properties of elements are the periodic functions of their ........................
atomic weight | |
mass number | |
atomic number | |
neutron number |
Question 26 |
Second group of elements are called ....................
alkali metals | |
alkaline earth metals | |
transition elements | |
minor transition elements |
Question 27 |
The ore FeS2 of iron is named as .....................
red haematite | |
iron pyrites | |
magnetite | |
cuprite |
Question 28 |
Air and water are necessary for ................... of iron.
oxidation | |
reduction | |
decomposition | |
rusting |
Question 29 |
For making electromagnets, ................... is used.
pig iron | |
wrought iron | |
steel | |
amalgam |
Question 30 |
In the correction of iron, carbonic acid acts as ......................
electrolyte | |
cathode | |
anode | |
an angent |
Question 31 |
.................. alloy of aluminium is used to make scientific instruments.
Magnalium | |
Duralumin | |
Brass | |
Bronze |
Question 32 |
Galena is the sulphide ore of ......................
aluminium | |
iron | |
copper | |
lead |
Question 33 |
............. is the substance added to the ore to reduce the fusion temperature.
flux | |
slag | |
gangue | |
mineral |
Question 34 |
The metal ................ plays a vital role in nuclear reactions releasing enormous energy called nuclear energy.
copper | |
chromium | |
uranium | |
zirconium |
Question 35 |
The ore of Aluminium is ..................
Haematite | |
Magnetite | |
Bauxite | |
Siderite |
Question 36 |
Bauxite is the ore of .................
Aluminium | |
Sodium | |
Copper | |
Iron |
Question 37 |
First period contains only two elements, one is hydrogen and the other is .............
Nitrogen | |
Oxygen | |
Helium | |
Neon |
Question 38 |
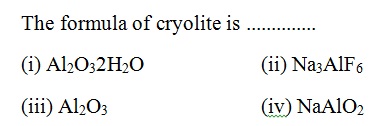
A | |
B | |
C | |
D |
Question 39 |

A | |
B | |
C | |
D |
Question 40 |
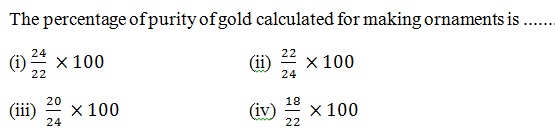
A | |
B | |
C | |
D |
Question 41 |
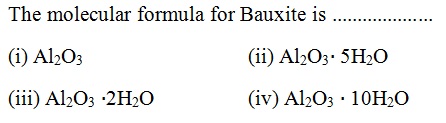
A | |
B | |
C | |
D |
Once you are finished, click the button below. Any items you have not completed will be marked incorrect.
There are 41 questions to complete.